Editor's Note. This is the first installment of a three-part series on the discovery of a cryptic pleurocerid species in East Tennessee. The present essay was published in the FMCS Newsletter Ellipsaria 18(2): 15 - 16 [pdf] if you're looking for something citable.
"endless forms most beautiful and most wonderful have been, and are being, evolved."
I like to
imagine that, as Charles Darwin wrote the poetic final clause of his
masterwork, he may have been gazing over some well-curated systematic
collection of molluscan shells [1].
Shells are captivating things, aren’t they?
This month’s installment in my long-running fascination
with The Shell began in 2007, when I first glimpsed the phenomenon we ultimately
christened “cryptic phenotypic plasticity" [2]. Using a survey of gene frequencies at a pair of highly polymorphic allozyme-encoding
loci, John Robinson and I had just discovered that the pleurocerid populations
we were identifying as Goniobasis acutocarinata, Goniobasis clavaeformis, and
Pleurocera unciale on the basis of their strikingly different shell
morphologies apparently constituted a single biological species [3]. Our samples had come from Indian Creek, a tributary
of the Powell River on the Virginia/Tennessee line. And I was curious to see if these intriguing
results might extend generally through East Tennessee and into North Georgia,
implying that the shell morphological distinction historically used to
distinguish the pleurocerid genera Goniobasis/Elimia from Pleurocera might have
no heritable basis.
So I picked three fresh study areas – the Little River drainage
in the vicinity of Maryville (TN), the Conasauga drainage east of Etowah (TN), and
the Coahulla near Dalton (GA). And from
each of these three regions I sampled three populations from the
acutocarinata-clavaeformis-unciale-carinifera continuum to analyze together
with my original data set from the Powell drainage. And from each of these (now four) regions I also
needed a control – a population of some common, widespread pleurocerid that
everybody recognizes, the specific status of which nobody doubts.
Populations of Pleurocera simplex are very nearly
omnipresent in small streams throughout Southwest Virginia, East Tennessee, and
North Georgia. They are also rather
distinctive, with their dark, smooth, teardrop-shaped shells and strikingly
black bodies. Thomas Say described
“Melania” simplex in 1825 from “a stream running from Abingdon to the Salt
Works, and from the stream on which General Preston’s grist-mill is situated,
as well as in a brook running through the salt water valley and discharging
into the Holstein River.” The eighteenth-century
salt mines to which Say must have been referring are still identifiable in
modern day Saltville (VA), and (indeed) snail populations matching Say’s small
figure and description still inhabit streams in the vicinity. And Say’s nomen “simplex” is among the oldest
names available for any American pleurocerid [4], so is in no danger of being
synonymized under anything else.
I included two populations of Pleurocera simplex (as
“Goniobasis simplex”) in the first allozyme study I ever published, way back in
1980 [5], and added five fresh simplex populations to control that 2007
gray-literature report with John Robinson I referenced to open the present
essay, extending west from the Saltville type locality across the Holston, Clinch
and Powell drainages of SW Virginia [6].
Clearly Pleurocera simplex would be the perfect control for my follow-up
study of the acutocarinata-clavaeformis-unciale-carinifera continuum now
extending south, through East Tennessee and into North Georgia. Am I right?
And so it was that on 7May08, I came to stand on the
banks of Pistol Creek in Courthouse Park, Maryville. What a lovely little city is Maryville,
Tennessee! Spreading like a cool oasis
between the county courthouse and historic Maryville College I found a shady
park full of happy picnickers and laughing children. And Pistol Creek is chock full of Pleurocera
clavaeformis acutocarinata, which was the focus of my attention on that sunny spring
afternoon, mixed with a population of good old familiar Pleurocera simplex, the
perfect control. I was probably there no
more than 30 minutes. In my field notes
I wrote, “Very pretty spot! Friendly
girls drove me off.”
The genetic analysis would, of course, take somewhat
longer. I started running the gels for
the study that was ultimately published as “Robust shell phenotype is a local
response to stream size in the genus Pleurocera” [7] later that month, extending
through the summer. I pulled the first
sample of 7 individuals from the bag labelled “Maryville simplex S6” on July 9,
with another 15 individuals on July 11.
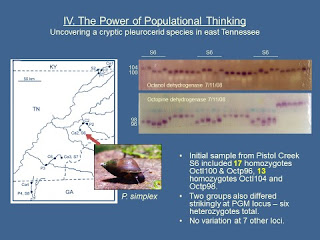
The PowerPoint slide below [8] shows photos of two of the nine gels I
ran on 7/11/08 – one stained for octanol dehydrogenase (Octl), the other
stained for octopine dehydrogenase (Octp).
The 15 Maryville simplex (labeled “S6”) were interleaved that day with
ten simplex individuals from someplace else and six P. clavaeformis – don’t
worry about those other 16 samples.
Much to my wondering eyes, the 15 “simplex” from
Maryville S6 proved to comprise 9 homozygotes for Octl104 and Octp98, and 6
homozygotes for Octl100 and Octp96, with no heterozygotes in evidence at either
locus. There were also striking
differences between the set of 9 and the set of 6 at the PGM locus. I wrote in my lab notebook, “News
Flash!! There are TWO species inside
Maryville S6. Criminy!”
Over the course of the next several runs, the sample of
30 snails I had collected from Maryville on the afternoon of 7May08 ultimately proved
to include 17 of the one species and 13 of the other. And almost immediately an important follow-up
question began to nag me. Might there have
been some subtle morphological difference between these two sets of snails I
had initially lumped together as Pleurocera simplex? What about the shells? Were they absolutely indistinguishable? Alas, I had cracked the shells and thrown
them all away when I froze my “Maryville simplex S6” sample back in May, following
my standard practice.
So in August of
2008 I returned to Maryville Courthouse Park for a second sample. And on this second visit, I examined the shells
much more critically. Standing ankle-deep in Pistol Creek, I developed the impression
that significant variation might exist in the simple shell proportions of the
snails I had previously lumped together as P. simplex, particularly with
respect to the relative heights of their body whorls. Although the differences were slight – indeed
negligible in juveniles and subadults – it seemed possible to me that the creek
might be inhabited by an admixture of two snail populations with dark, smooth,
teardrop-shaped shells and strikingly black bodies, one with “fat” shells and
the other with “skinny.”
So, for the 71 Maryville snails I carried back to
Charleston the next day, I measured the maximum shell dimension (or "shell
height"), and body whorl height (B), defined as the length of the final
360⁰ of whorl, along the axis of coiling. I then defined apex height (A) as
shell height minus body whorl height, and analyzed the relationship between
body whorl height and apex height by analysis of covariance using the separate
slopes model (JMP version 7). I then
classified my fresh sample of 71 individuals by their phenotype at 10 allozyme-encoding
loci using standard methods.
A total of 20 snails proved homozygous for Oldh100, while
51 were homozygous for Oldh104, with no putative heterozygotes again in
evidence. Differences were also very marked at the Opdh and PGM loci, although
a few heterozygotes were observed in both groups. The combined sample of 20 August snails plus
17 snails from the May sample showed Opdh96 = 0.946 and Pgm96 = 0.946, and the
combined sample of 51 + 13 showed Opdh98 = 0.953 and Pgm102 = 0.852. Again no variation was detected at the seven
additional genetic loci examined.
The figure above compares the regressions of (A) on (B)
for the two subsamples, the N = 20 fixed for Oldh100 and the N = 51 fixed for
Oldh104. Sure enough, the regressions
are quite significantly different [9]. I
provisionally designated the (N = 20) snails fixed for Oldh100 as population
S6f, with f appended for “fat,”, and the (N = 51) snails fixed for Oldh104 as
population S6s, for “skinny.”
One of these populations must surely match Thomas Say’s
bona fide simplex from that “brook running through the salt water valley and
discharging into the Holstein River,” I should hope! But which one? And what might be the identity of the other
population? Tune in next time!
Notes
[2] I originally christened the phenomenon “Goodrichian
Taxon Shift” in February of 2007, focusing entirely upon freshwater snails. Stephen Jacquemin, Mark Pyron, and I
broadened the concept into “cryptic phenotypic plasticity” in a (2013) paper we
published in Hydrobiologia [PDF]. Most of the
(now 17) blog posts filed under “Phenotypic Plasticity” in the blog index at
right above touch upon the pervasive phenomenon of CPP in freshwater gastropod
shells. But see particularly:
[4] Thomas Say published four pleurocerid names in 1825:
simplex, proxima, subglobosa, and fluvialis.
There are eight older pleurocerid names in the literature: virginica
(Gmelin 1791), carinata (Brug. 1792), verrucosa (Say 1820), armigera (Say
1821), canaliculata (Say 1821), praerosa (Say 1821), catenaria (Say 1822), and
carinifera (Lam. 1822).
[5] Dillon, R.T. and G.M. Davis (1980) The Goniobasis of
southern Virginia and northwestern North Carolina: Genetic and shell
morphometric relationships. Malacologia 20: 83-98. [PDF]
[6] Dillon, R. T. & J. D. Robinson (2007a) The Goniobasis ("Elimia") of
southwest Virginia, I. Population
genetic survey. Report to the Virginia
Division of Game and Inland Fisheries.
25 pp. [PDF]
[7] Dillon, R. T. (2011)
Robust shell phenotype is a local response to stream size in the genus
Pleurocera (Rafinesque 1818). Malacologia 53: 265-277. [PDF] I featured these results in two blog posts:
Dillon & Robinson (2011) When Cometh Our
Reformation? Molecular typology meets
population genetics in the pleurocerid gastropod fauna of east Tennessee.